Rumex acetosella
Sp. Pl. 1: 338. 1753.
Plants perennial, glabrous, with vertical rootstock and/or creeping rhizomes. Stems erect or ascending, several from base, branched in distal 1/2 (in inflorescence), 10–40(–45) cm; shoots variable. Leaves: ocrea brownish at base, silvery and lacerated in distal 1/2; blade normally obovate-oblong, ovate-lanceolate, lanceolate-elliptic, or lanceolate, occasionally, linear-lanceolate to almost linear, 2–6 × 0.3–2 cm, base hastate (with spreading, entire or sometimes multifid, dissected lobes), occasionally without evident lobes, then base broadly cuneate, margins entire, flat or nearly so, apex acute or obtuse. Inflorescences terminal, usually occupying distal 1/2–2/3 of stem, usually lax and interrupted to top, broadly or narrowly paniculate. Pedicels 1–3 mm. Flowers (3–)5–8(–10) in whorls; inner tepals not or slightly enlarged, normally 1.2–1.7(–2) × 0.5–1.3 mm (free wing absent or barely visible), base cuneate, apex obtuse or subacute. Achenes brown or dark brown, 0.9–1.5 × 0.6–0.9 mm. 2n = 14, 28, 42.
Phenology: Flowering spring–summer.
Habitat: Roadsides, cultivated fields, waste places, disturbed areas, lawns, meadows, railroad gravels, sandy and muddy shores: usually in acidic soils
Elevation: 0-2700 m
Distribution
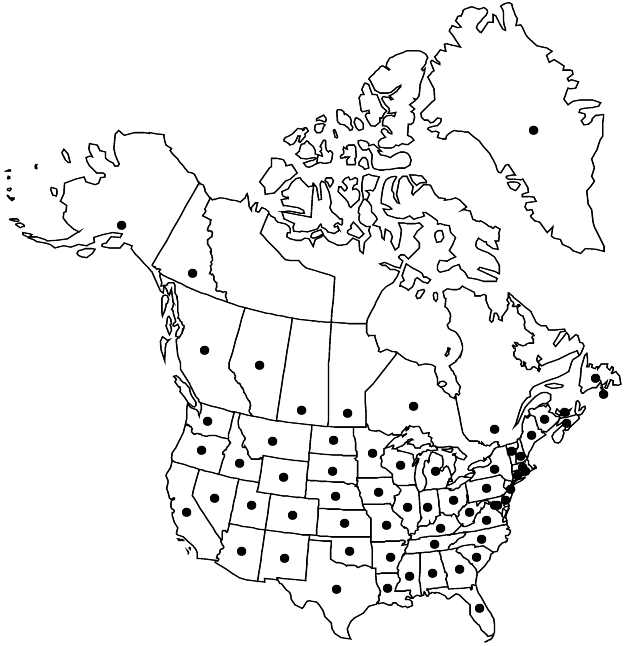
Introduced; Greenland, St. Pierre and Miquelon, Alta., B.C., Man., N.B., Nfld. and Labr. (Nfld.), N.S., Ont., P.E.I., Que., Sask., Yukon, Ala., Alaska, Ariz., Ark., Calif., Colo., Conn., Del., D.C., Fla., Ga., Idaho, Ill., Ind., Iowa, Kans., Ky., La., Maine, Md., Mass., Mich., Minn., Miss., Mo., Mont., Nebr., Nev., N.H., N.J., N.Mex., N.Y., N.C., N.Dak., Ohio, Okla., Oreg., Pa., R.I., S.C., S.Dak., Tenn., Tex., Utah, Vt., Va., Wash., W.Va., Wis., Wyo., Europe, w Asia, introduced almost worldwide.
Discussion
Rumex acetosella in the broad sense is an extremely variable and taxonomically complicated polyploid complex, which includes diploids, tetraploids, hexaploids, and octoploids. This complex (excluding more distantly related arctic-montane R. graminifolius and its allies) probably originated and developed mostly in southern Europe and southwestern Asia. Some races of R. acetosella now are distributed almost worldwide as introduced and often completely naturalized aliens.
Á. Löve (1941, 1983) assumed that in this group chromosome numbers are strictly correlated with morphology. In his opinion, every chromosome race represents a distinct species: diploid Rumex angiocarpus Murbeck [= Acetosella angiocarpa (Murbeck) Á. Löve]; tetraploid R. multifidus Linnaeus [= R. tenuifolius (Wallroth) Á. Löve = Acetosella multifida (Linnaeus) Á. Löve]; hexaploid R. acetosella in the narrow sense [= A. vulgaris (W. D. J. Koch) Fourreau, with gymnocarpous A. vulgaris subsp. vulgaris and angiocarpous A. vulgaris subsp. pyrenaica (Pourret ex Lapeyrouse) Á. Löve]; hexaploid R. graminifolius Rudolph ex Lambert [= A. graminifolia (Rudolph ex Lambert) Á. Löve]. However, the distribution given by Löve for these taxa seems unnatural. Studies by J. C. M. den Nijs and collaborators (den Nijs 1974, 1976, 1984; den Nijs and T. Panhorst 1980; den Nijs et al. 1980, 1985; see also W. Harris 1969, 1973) indicate that the situation is more complicated. They postulated the development of two major evolutionary lines into two ploidy complexes: a primary western Mediterranean one and a secondary eastern Mediterranean one. According to this scheme, polyploid races independently and spontaneously emerged (and still are emerging) within different ancestral populations.
The most widespread, almost cosmopolitan race, presumably native to the southwestern Mediterranean region, including southwestern and Atlantic Europe, which is common in North America, is characterized by a hexaploid chromosome set (2n = 42), nonmultifid lateral lobes of basal leaves, and angiocarpy (fruits are not easily separable from accrescent inner tepals). It was commonly and erroneously referred to as Rumex angiocarpus Murbeck, or R. acetosella subsp. angiocarpus (Murbeck) Murbeck. According to J. R. Akeroyd (1991), who in general followed the taxonomic revision of the group by J. C. M. den Nijs (1984), the correct name for this taxon is R. acetosella subsp. pyrenaicus (Pourret ex Lapeyrouse) Akeroyd (=Acetosella vulgaris subsp. pyrenaica (Pourret ex Lapeyrouse) Á. Löve). Gymnocarpous nonmultifid and multifid forms (R. acetosella subsp. acetosella and R. acetosella subsp. acetoselloides (Balansa) den Nijs, respectively) also occur in North America, but evidently rarely. The distributions of subspecies of R. acetosella in North America are poorly known. Keys and detailed descriptions for the subspecies were provided by den Nijs and Akeroyd. However, the tempting simplicity of the keys is somewhat suspicious. The alternative point of view (and an alternative key) may be found in Á. Löve (1983).
Rumex acetosella subsp. arenicola Mäkinen ex Elven was recently described from Greenland and reported for Scandinavia and arctic Russia (R. Elven et al. 2000). This entity seems to be morphologically transitional toward Rumex graminifolius (see discussion under that species below). According to Elven et al., it differs from other infraspecific entities of R. acetosella in having the following characters: leaves usually without basal lobes (as in R. graminifolius), with revolute margins; inflorescence sparsely branched; tepals and pedicels densely covered with red papillae (as in R. graminifolius). From R. graminifolius and related taxa (R. beringensis and R. krausei) it can be distinguished by narrower inner tepals (similar in size to those in other subspecies of R. acetosella). The distribution of subsp. arenicola and its relations to other taxa are in need of further study.
Selected References
None.
