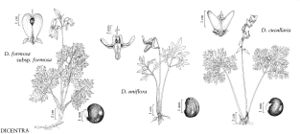
Dicentra cucullaria
Linnaea 8: 457, 468. 1833.
Plants perennial, scapose, from short rootstocks bearing pink to white, teardrop-shaped bulblets. Leaves (10-)14-16(-36) × (4-)6-14(-18) cm; petiole (5-)8-16(-24) cm; blade with 4 orders of leaflets and lobes; abaxial surface glaucous; ultimate lobes linear to linear-elliptic or linear-obovate, (2-)5-15(-23) × (0.4-)2-3(-4.2) mm, usually minutely apiculate. Inflorescences racemose, 3-14-flowered, usually exceeding leaves; bracts minute. Flowers pendent; pedicels (2-)4-7(-12) mm; sepals broadly ovate, 1.8-5 × 1.3-4 mm; petals white, frequently suffused pink, apex yellow to orange-yellow; outer petals (10-)12-16(-20) × (3-)6-10(-13) mm, reflexed portion 2-5 mm; inner petals (7.5-)9-12(-14) mm, blade 1.8-4 mm, claw linear, 4-8 × less than 1 mm, crest prominent, ca. 2 mm diam.; filaments of each bundle connate from base to shortly below anthers; nectariferous tissue forming 1-3(-4.5) mm spur diverging at angle from base of bundle; style 2-4 mm; stigma 2-horned with 2 lateral papillae. Capsules ovoid, attenuate at both ends, (7-)9-13(-16) × 3-5 mm. Seeds reniform, ca. 2 mm diam., very obscurely reticulate, elaiosome present. 2n = 32.
Phenology: Flowering early–late spring.
Habitat: Deciduous woods and clearings, in rich loam soils
Elevation: 0-1500 m
Distribution
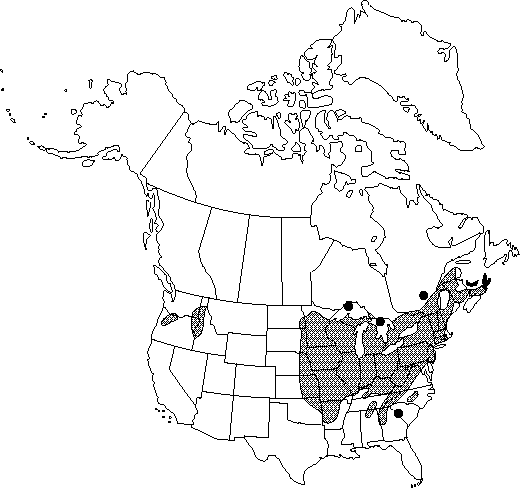
N.B., N.S., Ont., P.E.I., Que., Ala., Ark., Conn., Del., D.C., Ga., Idaho, Ill., Ind., Iowa, Kans., Ky., Maine, Md., Mass., Mich., Minn., Mo., Nebr., N.H., N.J., N.Y., N.C., N.Dak., Ohio, Okla., Oreg., Pa., R.I., S.C., S.Dak., Tenn., Vt., Va., Wash., W.Va., Wis.
Discussion
Dicentra cucullaria is occasionally confused with D. canadensis, with which it is sympatric. It is distinguished from that species by its basally pointed (versus rounded) outer petal spurs, by its flowers lacking a fragrance, by flowering 7-10 days earlier, and by its pink to white, teardrop-shaped (versus yellow, pea-shaped) bulblets.
After fruit set, the bulblets of both Dicentra cucullaria and D. canadensis remain dormant until fall, when stored starch is converted to sugar. At this time also, flower buds and leaf primordia are produced below ground; these then remain dormant until spring (P. G. Risser and G. Cottam 1968; B. J. Kieckhefer 1964; K. R. Stern 1961). Pollination of both species is effected by bumblebees (Bombus spp.) and other long-tongued insects (L. W. Macior 1970, 1978; K. R. Stern 1961).
Flavonoid components indicate that Dicentra canadensis and D. cucullaria are more closely related to each other than to any other member of the genus (D. Fahselt 1971). Even so, species purported to be hybrids between them probably are not. There is considerable variation in floral morphology within D. cucullaria, which can have flowers superficially resembling those of D. canadensis. However, when all characters of the plants are examined, these putative hybrids almost always are clearly assignable to one species or the other.
The western populations of Dicentra cucullaria appear to have been separated from the eastern ones for at least a thousand years. The western plants are generally somewhat coarser, which apparently led Rydberg to designate the western populations as a separate species. Plants from the Blue Ridge Mountains of Virginia, however, are virtually indistinguishable from those of the West, and much of the variation (which is considerable) within the species probably involves phenotypic response to the environment, or represents ecotypes within the species.
The Iroquois prepared infusions from the roots of Dicentra cucullaria for a medicinal liniment (D. E. Moerman 1986).
Selected References
None.