Difference between revisions of "Polypodium hesperium"
Proc. Biol. Soc. Wash. 13: 200. 1900.
FNA>Volume Importer |
FNA>Volume Importer |
||
| Line 60: | Line 60: | ||
|publication year=1900 | |publication year=1900 | ||
|special status= | |special status= | ||
| − | |source xml=https://jpend@bitbucket.org/aafc-mbb/fna-data-curation.git/src/ | + | |source xml=https://jpend@bitbucket.org/aafc-mbb/fna-data-curation.git/src/f6b125a955440c0872999024f038d74684f65921/coarse_grained_fna_xml/V2/V2_156.xml |
|genus=Polypodium | |genus=Polypodium | ||
|species=Polypodium hesperium | |species=Polypodium hesperium | ||
Revision as of 18:55, 24 September 2019
Stems occasionally whitish pruinose, slender to moderately stout, to 6 mm diam., acrid- to sweet-tasting: scales concolored, brown or slightly mottled, often darker near point of attachment, lanceolate, usually symmetric, margins entire to denticulate. Leaves to 35 cm. Petiole slender, to 1.5 mm diam. Blade oblong to lanceolate-ovate, occasionally deltate, pinnatifid, usually widest at or near middle, to 7 cm wide, herbaceous to somewhat leathery; rachis sparsely scaly to glabrescent abaxially, glabrous adaxially; scales linear-lanceolate, usually less than 6 cells wide. Segments oblong to linear-lanceolate, less than 12 mm wide; margins entire to crenulate or obscurely serrate; apex obtuse to acute; midrib glabrous adaxially. Venation free. Sori midway between margin and midrib, less than 3 mm diam., oval when immature. Sporangiasters absent. Spores more than 58 µm, rugose to verrucose or tuberculate, surface projections commonly less than 3 µm. 2n = 148.
Phenology: Sporulating summer–fall.
Habitat: Cracks and ledges on cliffs, on a variety of noncalcareous substrates, rarely on limestone
Elevation: 300–3500 m.
Distribution
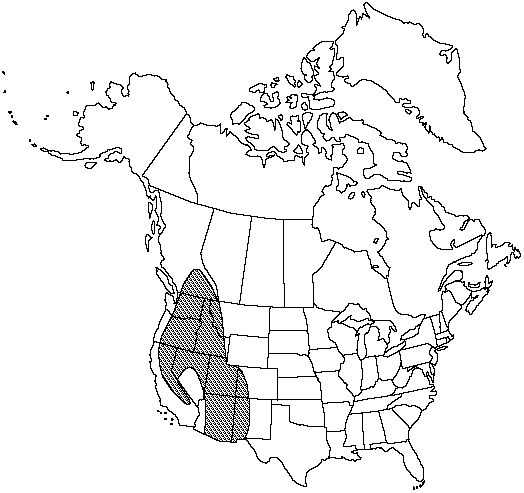
B.C., Ariz., Calif., Colo., Idaho, Mont., Nev., N.Mex., Oreg., Utah, Wash., Mexico in Chihuahua, Baja California.
Discussion
Using morphologic and chromosomal data, F. A. Lang (1971) proposed that Polypodium hesperium originated through allotetraploidy involving P. glycyrrhiza and P. amorphum, a hypothesis recently supported by electrophoretic studies (C. H. Haufler, M. D. Windham, and E. W. Rabe, unpublished). Variations in spore surface morphology and banding patterns observed in isozyme studies indicate that P. hesperium may have originated more than once from different individuals of the same species. Some collections of P. hesperium can be mistaken for P. glycyrrhiza, but the latter species is easily distinguished by its pubescent rachises, linear blade scales, and smaller spores (less than 58 µm). Although P. amorphum has sporangiasters and P. hesperium lacks them, misshapen sporangia in P. hesperium can mimic these distinctive soral structures. Therefore, it is often necessary to use a combination of soral, stem scale, and blade scale features (discussed in the key) to separate P. hesperium from P. amorphum. Hybridization occurs between P. hesperium and each of its progenitor diploids to form triploid individuals with misshapen spores (F. A. Lang 1971). Rare, sterile, tetraploid hybrids with P. saximontanum have also been detected (M. D. Windham, unpublished).
Selected References
None.