Difference between revisions of "Arceuthobium campylopodum"
Boston J. Nat. Hist. 6: 214. 1850.
FNA>Volume Importer |
imported>Volume Importer |
||
| Line 28: | Line 28: | ||
-->{{Treatment/Body | -->{{Treatment/Body | ||
| − | |distribution= | + | |distribution=B.C.;Alaska;Ariz.;Calif.;Colo.;Idaho;Mont.;N.Mex.;Nev.;Oreg.;Utah;Wash.;Wyo.;n Mexico. |
|discussion=<p>Subspecies 13 (13 in the flora).</p><!-- | |discussion=<p>Subspecies 13 (13 in the flora).</p><!-- | ||
--><p><i>Arceuthobium campylopodum</i> is here considered a wide ranging and polymorphic species that parasitizes a number of hosts in Pinaceae throughout western North America and northern Mexico. Earlier taxonomic concepts, such as those of L. S. Gill (1935) and J. Kuijt (1955), recognized several forms of <i>A. campylopodum</i> that usually corresponded with principal host species. F. G. Hawksworth and D. Wiens (1972, 1996) elevated those forms to species, stating that morphologic integrity is maintained even when parasitizing nonprincipal hosts. Despite this claim, most of the characters they used to differentiate species were quantitative with continuous variation. The absence of hybridization was also given as evidence for separate species status; however, all species have the same chromosome number and very similar morphology, thus it is not clear how a hybrid would be recognized should it occur. Examination of dwarf mistletoe specimens without information on location and host can often result in an ambiguous identification.</p><!-- | --><p><i>Arceuthobium campylopodum</i> is here considered a wide ranging and polymorphic species that parasitizes a number of hosts in Pinaceae throughout western North America and northern Mexico. Earlier taxonomic concepts, such as those of L. S. Gill (1935) and J. Kuijt (1955), recognized several forms of <i>A. campylopodum</i> that usually corresponded with principal host species. F. G. Hawksworth and D. Wiens (1972, 1996) elevated those forms to species, stating that morphologic integrity is maintained even when parasitizing nonprincipal hosts. Despite this claim, most of the characters they used to differentiate species were quantitative with continuous variation. The absence of hybridization was also given as evidence for separate species status; however, all species have the same chromosome number and very similar morphology, thus it is not clear how a hybrid would be recognized should it occur. Examination of dwarf mistletoe specimens without information on location and host can often result in an ambiguous identification.</p><!-- | ||
| Line 159: | Line 159: | ||
|basionyms= | |basionyms= | ||
|family=Viscaceae | |family=Viscaceae | ||
| − | |distribution= | + | |distribution=B.C.;Alaska;Ariz.;Calif.;Colo.;Idaho;Mont.;N.Mex.;Nev.;Oreg.;Utah;Wash.;Wyo.;n Mexico. |
|reference=mathiasen2015a;nickrent2012a;reif2015a | |reference=mathiasen2015a;nickrent2012a;reif2015a | ||
|publication title=Boston J. Nat. Hist. | |publication title=Boston J. Nat. Hist. | ||
|publication year=1850 | |publication year=1850 | ||
|special status=Illustrated | |special status=Illustrated | ||
| − | |source xml=https:// | + | |source xml=https://bibilujan@bitbucket.org/aafc-mbb/fna-data-curation.git/src/bb6b7e3a7de7d3b7888a1ad48c7fd8f5c722d8d6/coarse_grained_fna_xml/V12/V12_560.xml |
|genus=Arceuthobium | |genus=Arceuthobium | ||
|species=Arceuthobium campylopodum | |species=Arceuthobium campylopodum | ||
Revision as of 20:10, 27 May 2020
Plants forming localized infections only or nonsystemic witches' brooms. Stems yellow, yellowish green, green, olive green, brown, light tan, orange, red, maroon, or purple; secondary branching fanlike, branches 3–10(–22) cm, third internode 2–15(–23) × 1–3.5(–5) mm, dominant shoot 1–6 mm diam. at base. Staminate pedicels absent. Staminate flowers slightly asymmetric (distal lateral petals keeled and hooded, proximal petal not keeled, deflexed at anthesis), lenticular in bud, 2.3–3.3 mm diam.; petals 3–4(–6), yellowish green. Berries proximally yellowish green or olive green, distally yellow, orange, or brown, 3–5 × 2–3 mm. Seeds pyriform to ellipsoid, 3.3–5.5 × 2.3–4 mm, endosperm bright green. 2n = 28.
Distribution
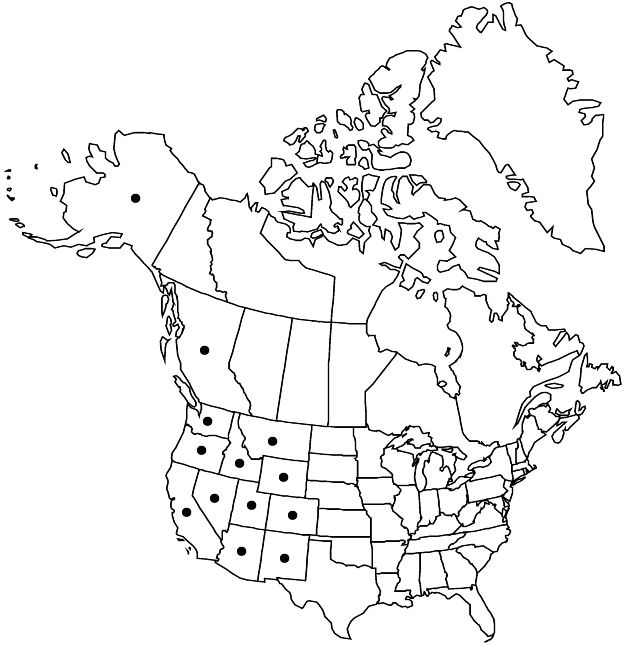
B.C., Alaska, Ariz., Calif., Colo., Idaho, Mont., N.Mex., Nev., Oreg., Utah, Wash., Wyo., n Mexico.
Discussion
Subspecies 13 (13 in the flora).
Arceuthobium campylopodum is here considered a wide ranging and polymorphic species that parasitizes a number of hosts in Pinaceae throughout western North America and northern Mexico. Earlier taxonomic concepts, such as those of L. S. Gill (1935) and J. Kuijt (1955), recognized several forms of A. campylopodum that usually corresponded with principal host species. F. G. Hawksworth and D. Wiens (1972, 1996) elevated those forms to species, stating that morphologic integrity is maintained even when parasitizing nonprincipal hosts. Despite this claim, most of the characters they used to differentiate species were quantitative with continuous variation. The absence of hybridization was also given as evidence for separate species status; however, all species have the same chromosome number and very similar morphology, thus it is not clear how a hybrid would be recognized should it occur. Examination of dwarf mistletoe specimens without information on location and host can often result in an ambiguous identification.
During the 1980s and 1990s, biosystematic studies were conducted using isozyme electrophoresis, particularly within sect. Campylopoda. Results of these studies, summarized by D. L. Nickrent (1996), in some cases provided evidence for populational genetic divergence but in other cases did not. Following those studies, phylogenetic analyses of chloroplast trnL-F and nuclear rDNA ITS sequences were applied to species level questions (Nickrent et al. 1994, 2004). All members of sect. Campylopoda had essentially identical DNA sequences in these regions, in contrast with species in other sections, which showed greater genetic distances.
All taxa within sect. Campylopoda were examined with respect to their morphology, host associations, levels of sympatry, and genetic relationships (D. L. Nickrent 2012). That study concluded that all taxa of sect. Campylopoda, recognized as species by F. G. Hawksworth and D. Wiens (1996), are best viewed as ecotypes of a single variable species, A. campylopodum. Two factors were used to justify describing the variants at the rank of subspecies: less than 20% of the time are the 13 subspecies sympatric with one another (they are generally geographically and ecologically isolated), and subspecies have already been described in A. vaginatum. For a complete list of synonyms for each subspecies, see Nickrent.
A morphometric study using discriminant function analysis was conducted by R. L. Mathiasen and S. C. Kenaley (2015) on four taxa within sect. Campylopoda: A. campylopodum subspp. campylopodum, littorum, occidentale, and siskiyouense. With the exception of male plants of the last subspecies, this analysis showed that the 95% confidence intervals overlapped, indicating potential misclassification of the taxa assigned a priori to species. Differences in multivariate means indicate that some differentiation has occurred among these four taxa. An additional three taxa of sect. Campylopdoda that parasitize white pines were examined by B. P. Reif et al. (2015) using amplified fragment length polymorphisms: A. campylopodum subspp. apachecum, cyanocarpum, and blumeri. Support was low for genetic differentiation between the first two subspecies whereas greater differentiation was seen between these and the third, in agreement with D. L. Nickrent et al. (2004). These two studies examined seven of the 13 taxa in sect. Campylododa, hence more work is needed simultaneously treating the entire complex. Although these authors argued that their results support classification at the species level, their results could also be viewed as evidence for early stages of genetic differentiation among widespread populations, thus equally supporting classification at the subspecific rank.
Selected References
Lower Taxa
Key
| 1 | Stems 3–5(–11) cm; principal hosts Larix, Picea, Pinus albicaulis, P. aristata, P. flexilis, P. longaeva, P. strobiformis, and Tsuga; plants forming witches' brooms; usually occurring at 1000–3000 m elevation. | > 2 |
| 2 | Stems 3(–9) cm; principal host Pinus. | > 3 |
| 3 | Third internodes 5–7.2(–10) mm; staminate flowers 2.7 mm diam.; principal host Pinus strobiformis; Arizona, New Mexico. | Arceuthobium campylopodum subsp. apachecum |
| 3 | Third internodes 2–5.2(–14) mm; staminate flowers 3 mm diam.; principal hosts Pinus albicaulis, P. aristata, P. flexilis, and P. longaeva; widely distributed in w United States but not Arizona. | Arceuthobium campylopodum subsp. cyanocarpum |
| 2 | Stems 4–5(–11) cm; principal hosts Larix, Picea, and Tsuga. | > 4 |
| 4 | Staminate flowers 2.7 mm diam.; principal hosts Larix and Tsuga; British Columbia, nw United States. | Arceuthobium campylopodum subsp. laricis |
| 4 | Staminate flowers 2.3 mm diam.; principal host Picea; Arizona, New Mexico. | Arceuthobium campylopodum subsp. microcarpum |
| 1 | Stems 5–12(–22) cm; principal hosts Abies, Pinus attenuata, P. contorta, P. jeffreyi, P. lambertiana, P. monticola, P. muricata, P. ponderosa, P. radiata, P. sabiniana, P. strobiformis, and Tsuga; plants forming localized infections only or forming witches' brooms; usually occurring at 0–2500 m elevation. | > 5 |
| 5 | Principal hosts Abies, Pinus contorta, and Tsuga. | > 6 |
| 6 | Stems 8(–22) cm; third internodes 4–14(–23) cm; principal hosts Abies concolor and A. magnifica; Arizona, California, Nevada, Oregon, Utah, Washington. | Arceuthobium campylopodum subsp. abietinum |
| 6 | Stems 5–7(–13) cm; third internodes 4–9(–16) cm; principal hosts Abies, Pinus, and Tsuga; British Columbia to California. | Arceuthobium campylopodum subsp. tsugense |
| 5 | Principal host Pinus (subsp. siskiyouense, rarely on Pinus contorta). | > 7 |
| 7 | Principal host Pinus strobiformis; plants usually forming localized infections only; Arizona. | Arceuthobium campylopodum subsp. blumeri |
| 7 | Principal hosts other than Pinus strobiformis; plants forming localized infections only or forming witches' brooms; California, Idaho, Oregon, Washington. | > 8 |
| 8 | Principal hosts Pinus subg. Strobus (white and soft pines). | > 9 |
| 9 | Shoots yellow or green; plants forming witches' brooms; principal host Pinus lambertiana; California. | Arceuthobium campylopodum subsp. californicum |
| 9 | Shoots olive green or brown; plants forming localized infections only; principal host Pinus monticola; nw California, sw Oregon. | Arceuthobium campylopodum subsp. monticola |
| 8 | Principal hosts Pinus subg. Pinus. | > 10 |
| 10 | Plants usually forming witches' brooms. | > 11 |
| 11 | Third internodes 7–11(–22) × 1.5–2(–2.5) mm; staminate flowers: petals 3(–4); principal hosts Pinus jeffreyi and P. ponderosa; California, Idaho, Oregon, Washington. | Arceuthobium campylopodum subsp. campylopodum |
| 11 | Third internodes 10–15(–20) × 2–3.5(–5) mm; staminate flowers: petals 4; principal hosts Pinus muricata and P. radiata; coastal California. | Arceuthobium campylopodum subsp. littorum |
| 10 | Plants usually forming localized infections only. | > 12 |
| 12 | Stems yellow or orange; principal host Pinus sabiniana; flowering Sep–Nov(–Dec); California (surrounding central valley and in Coast Ranges). | Arceuthobium campylopodum subsp. occidentale |
| 12 | Stems brown; principal host Pinus attenuata; flowering Aug–Sep; nw California, sw Oregon. | Arceuthobium campylopodum subsp. siskiyouense |