Difference between revisions of "Coryphantha missouriensis"
in N. L. Britton and A. Brown, Ill. Fl. N. U. S. ed. 2, 2: 570. 1913.
FNA>Volume Importer |
FNA>Volume Importer |
||
| Line 11: | Line 11: | ||
|name=Mammillaria missouriensis | |name=Mammillaria missouriensis | ||
|authority=Sweet | |authority=Sweet | ||
| + | |rank=species | ||
|publication_title=Hort. Brit., | |publication_title=Hort. Brit., | ||
|publication_place=171. 1826 | |publication_place=171. 1826 | ||
| Line 17: | Line 18: | ||
|name=Coryphantha missouriensis var. caespitosa | |name=Coryphantha missouriensis var. caespitosa | ||
|authority=(Engelmann) L. D. Benson | |authority=(Engelmann) L. D. Benson | ||
| + | |rank=variety | ||
}} {{Treatment/ID/Synonym | }} {{Treatment/ID/Synonym | ||
|name=Coryphantha missouriensis var. robustior | |name=Coryphantha missouriensis var. robustior | ||
|authority=(Engelmann) L. D. Benson | |authority=(Engelmann) L. D. Benson | ||
| + | |rank=variety | ||
}} {{Treatment/ID/Synonym | }} {{Treatment/ID/Synonym | ||
|name=Escobaria missouriensis | |name=Escobaria missouriensis | ||
|authority=(Engelmann) Britton & Rose | |authority=(Engelmann) Britton & Rose | ||
| + | |rank=species | ||
}} {{Treatment/ID/Synonym | }} {{Treatment/ID/Synonym | ||
|name=Neobesseya missouriensis | |name=Neobesseya missouriensis | ||
|authority=(Sweet) Britton & Rose ex Rydberg | |authority=(Sweet) Britton & Rose ex Rydberg | ||
| + | |rank=species | ||
}} {{Treatment/ID/Synonym | }} {{Treatment/ID/Synonym | ||
|name=Neobesseya similis | |name=Neobesseya similis | ||
| − | |authority= | + | |authority= |
| + | |rank=species | ||
}} {{Treatment/ID/Synonym | }} {{Treatment/ID/Synonym | ||
|name=Neomam-millaria missouriensis | |name=Neomam-millaria missouriensis | ||
| − | |authority= | + | |authority= |
| + | |rank=species | ||
}} | }} | ||
|hierarchy=Cactaceae;Cactaceae subfam. Cactoideae;Coryphantha;Coryphantha missouriensis | |hierarchy=Cactaceae;Cactaceae subfam. Cactoideae;Coryphantha;Coryphantha missouriensis | ||
| Line 49: | Line 56: | ||
--><p><i>Coryphantha missouriensis</i> and its allies are diploid, except for the populations long known as Neobesseya similis (Engelmann) Britton & Rose, in the southeastern portion of the species’ range. Presently too few chromosome counts are available to allow satisfactory classification of the polyploids, which are larger in all parts but not always identifiable from morphology alone. The polyploids include some male-sterile plants, perhaps indicating some form of dioecy; their pollen grains display as much variation as the rest of the genus combined. Plants from Kansas and Oklahoma are especially morphologically ambiguous.</p><!-- | --><p><i>Coryphantha missouriensis</i> and its allies are diploid, except for the populations long known as Neobesseya similis (Engelmann) Britton & Rose, in the southeastern portion of the species’ range. Presently too few chromosome counts are available to allow satisfactory classification of the polyploids, which are larger in all parts but not always identifiable from morphology alone. The polyploids include some male-sterile plants, perhaps indicating some form of dioecy; their pollen grains display as much variation as the rest of the genus combined. Plants from Kansas and Oklahoma are especially morphologically ambiguous.</p><!-- | ||
--><p>All parts including seeds, of <i>Coryphantha missouriensis</i> plants are generally larger in the Southwest (i.e., in Arizona) than northward. The neotenous populations in Navajo County, Arizona, are an exception, with the smallest individuals, shortest spines of the species. Both large and small extremes are diploid. The epithet marstonii [apparently based by L. D. Benson (1969) on <i>C. vivipara</i>] has been misapplied to the various Arizona and Utah populations of <i>C. missouriensis</i>, especially the peculiar plants in Navajo County, Arizona. In cultivation, the largest Arizona plants match dimensions of field-grown plants from proven tetraploid populations in central Texas; however, under the same greenhouse conditions the Texas plants become larger still, reaching the size of <i>C. sulcata</i>.</p><!-- | --><p>All parts including seeds, of <i>Coryphantha missouriensis</i> plants are generally larger in the Southwest (i.e., in Arizona) than northward. The neotenous populations in Navajo County, Arizona, are an exception, with the smallest individuals, shortest spines of the species. Both large and small extremes are diploid. The epithet marstonii [apparently based by L. D. Benson (1969) on <i>C. vivipara</i>] has been misapplied to the various Arizona and Utah populations of <i>C. missouriensis</i>, especially the peculiar plants in Navajo County, Arizona. In cultivation, the largest Arizona plants match dimensions of field-grown plants from proven tetraploid populations in central Texas; however, under the same greenhouse conditions the Texas plants become larger still, reaching the size of <i>C. sulcata</i>.</p><!-- | ||
| − | --><p>The fictional endemic Texas variety “< | + | --><p>The fictional endemic Texas variety “<i></i>var.<i> robustior</i>” [= Neobesseya wissmannii (Hildman ex K. Schumann) Britton & Rose] never corresponded to a populational entity. It evolved in the literature from robust individual plants in ordinary eastern populations, through misidentified plants of the sympatric <i>C. sulcata</i>, to inaccurate descriptions and maps based on mixtures of both species. Most published photographs captioned as “Neobesseya wissmannii” have portrayed misidentified plants of <i>C. sulcata</i>.</p><!-- |
--><p>The closest relatives of <i>Coryphantha missouriensis</i> are C. asperispina Boedeker of Mexico and <i>C. cubensis</i> Britton & Rose of Cuba. Those species collectively composed the segregate genus Neobesseya, now rarely accepted.</p><!-- | --><p>The closest relatives of <i>Coryphantha missouriensis</i> are C. asperispina Boedeker of Mexico and <i>C. cubensis</i> Britton & Rose of Cuba. Those species collectively composed the segregate genus Neobesseya, now rarely accepted.</p><!-- | ||
--><p>Environmental degradation—introduced fire ants, suburban development, brush encroachment following fire suppression, and sometimes over-grazing—has extirpated <i>Coryphantha missouriensis</i> from many of its historically known sites. However, the vigorous polyploids rapidly colonize some disturbed habitats along with weedy species of <i>Opuntia</i>.</p> | --><p>Environmental degradation—introduced fire ants, suburban development, brush encroachment following fire suppression, and sometimes over-grazing—has extirpated <i>Coryphantha missouriensis</i> from many of its historically known sites. However, the vigorous polyploids rapidly colonize some disturbed habitats along with weedy species of <i>Opuntia</i>.</p> | ||
| Line 60: | Line 67: | ||
-->{{#Taxon: | -->{{#Taxon: | ||
name=Coryphantha missouriensis | name=Coryphantha missouriensis | ||
| − | |||
|authority=(Sweet) Britton & Rose in N. L. Britton and A. Brown | |authority=(Sweet) Britton & Rose in N. L. Britton and A. Brown | ||
|rank=species | |rank=species | ||
| Line 74: | Line 80: | ||
|publication year=1913 | |publication year=1913 | ||
|special status= | |special status= | ||
| − | |source xml=https://jpend@bitbucket.org/aafc-mbb/fna-data-curation.git/src/ | + | |source xml=https://jpend@bitbucket.org/aafc-mbb/fna-data-curation.git/src/f50eec43f223ca0e34566be0b046453a0960e173/coarse_grained_fna_xml/V4/V4_425.xml |
|subfamily=Cactaceae subfam. Cactoideae | |subfamily=Cactaceae subfam. Cactoideae | ||
|genus=Coryphantha | |genus=Coryphantha | ||
Revision as of 21:40, 16 December 2019
Plants unbranched or profusely branched (eastern populations), forming clumps to 30+ cm diam., spine-bearing areoles with short white wool not obscuring basal portion of spine. Roots ± diffuse or short taproots, sometimes adventitious from bases of branches. Stems deep-seated in substrate, becoming flat-topped and nearly subterranean in winter, oblate or spheric to obconic, 2–8 × (1–)1.8–7.5(–10) cm; tubercles 5–21 × 3–6+ mm, soft; areolar glands absent; parenchyma not mucilaginous; druses small and spheric; pith 1/5–1/3 of lesser stem diam.; medullary vascular system usually absent. Spines 6–21 per areole, bright white, pale gray, or pale tan, weathering to gray or yellowish brown, dark brownish orange to pale brown or pale grayish pink tips present on all or only the largest; radial spines 6–20 per areole, moderately to tightly appressed, 4–16 × 0.07–0.35 mm, excluding pubescence; outer central spines 0(–2) per areole, if 1, erect, if 2, ascending-spreading, 9–18 mm; inner central spines 0–1 per areole, porrect (rarely ascending or erect), straight, 8–20 × 0.2–0.4 mm. Flowers nearly apical, 18–50 × 15–50 mm; outer tepals fringed (rarely entire); inner tepals 13–25 per flower, pale greenish yellow to yellow-green with midstripes of green or rose-pink to pale brown; outer filaments brighter pink than inner tepals, pinkish with greenish white bases, or uniformly whitish; anthers bright yellow; stigma lobes 3–7, green or yellowish, 1–5 mm. Fruits orange-red to scarlet, sometimes proximally carmine or nearly magenta, spheric to ellipsoid, 6.5–10 × 5–9 mm, slightly succulent but not juicy, the funiculi papillate; floral remnant weakly persistent, often lost through weathering. Seeds black, nearly spheric to short pyriform, 1.4–2.2 mm., deeply pitted, outer cell walls concave. 2n = 22, 44.
Phenology: Flowering Apr–Jun; fruiting Jan–Jun(-Jul).
Habitat: Plains, stony short-grass prairies, woodlands of ponderosa pine, pinyon, juniper, or Quercus gambelii, loamy places, often restricted to sedimentary rocks
Distribution
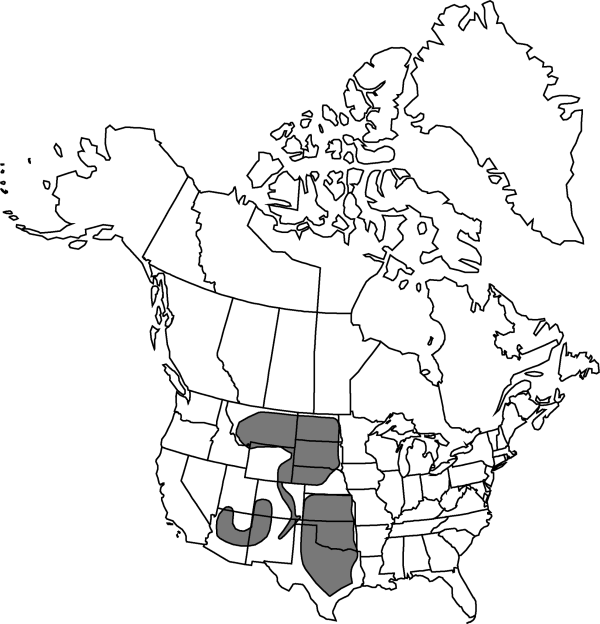
Ariz., Colo., Kans., Mont., Nebr., N.Mex., N.Dak., Okla., S.Dak., Tex., Utah, Wyo.
Discussion
Plants of Coryphantha missouriensis are localized, inconspicuous (except for their red fruits in spring), and difficult for non-specialists to distinguish from more common species. Reports of C. missouriensis from Idaho are based on misidentified specimens of Pediocactus simpsonii (in the broad sense). The record from southwestern New Mexico (L. D. Benson 1982) is based on immature Mammillaria heyderi var. bullingtoniana.
Coryphantha missouriensis and its allies are diploid, except for the populations long known as Neobesseya similis (Engelmann) Britton & Rose, in the southeastern portion of the species’ range. Presently too few chromosome counts are available to allow satisfactory classification of the polyploids, which are larger in all parts but not always identifiable from morphology alone. The polyploids include some male-sterile plants, perhaps indicating some form of dioecy; their pollen grains display as much variation as the rest of the genus combined. Plants from Kansas and Oklahoma are especially morphologically ambiguous.
All parts including seeds, of Coryphantha missouriensis plants are generally larger in the Southwest (i.e., in Arizona) than northward. The neotenous populations in Navajo County, Arizona, are an exception, with the smallest individuals, shortest spines of the species. Both large and small extremes are diploid. The epithet marstonii [apparently based by L. D. Benson (1969) on C. vivipara] has been misapplied to the various Arizona and Utah populations of C. missouriensis, especially the peculiar plants in Navajo County, Arizona. In cultivation, the largest Arizona plants match dimensions of field-grown plants from proven tetraploid populations in central Texas; however, under the same greenhouse conditions the Texas plants become larger still, reaching the size of C. sulcata.
The fictional endemic Texas variety “var. robustior” [= Neobesseya wissmannii (Hildman ex K. Schumann) Britton & Rose] never corresponded to a populational entity. It evolved in the literature from robust individual plants in ordinary eastern populations, through misidentified plants of the sympatric C. sulcata, to inaccurate descriptions and maps based on mixtures of both species. Most published photographs captioned as “Neobesseya wissmannii” have portrayed misidentified plants of C. sulcata.
The closest relatives of Coryphantha missouriensis are C. asperispina Boedeker of Mexico and C. cubensis Britton & Rose of Cuba. Those species collectively composed the segregate genus Neobesseya, now rarely accepted.
Environmental degradation—introduced fire ants, suburban development, brush encroachment following fire suppression, and sometimes over-grazing—has extirpated Coryphantha missouriensis from many of its historically known sites. However, the vigorous polyploids rapidly colonize some disturbed habitats along with weedy species of Opuntia.
Selected References
None.
